Close
Shop All:Innovating Science
Innovating Science Determination of Properties of Buffer Solutions AP Chemistry
About This Item
Description
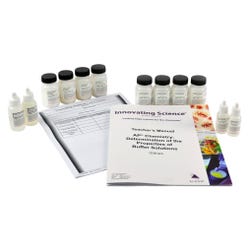
In this experiment, you will prepare three buffer solutions having different pH values and show that the pH of these solutions does not change significantly when small amounts of acids and bases are added. You will also show that when the same amounts of acids and bases are added to water and to a non-buffer solution (e.g. NaCl solution), the pH changes are large.Features
Includes:
-
1 x 30g Sodium Acetate, trihydrate
-
2 x 25g Ammonium Chloride
-
1 x 30g Sodium Bicarbonate
-
4 x 25g Sodium Chloride
-
1 x 23mL Acetic Acid EZ-Prep, to make 800mL of 0.5M Solution
-
1 x 27mL Ammonium Hydroxide, EZ-Prep, to make 800mL of 0.5M Solution
-
1 x 15mL Sodium Hydroxide, 1.0N Solution
-
1 x 15mL Hydrochloric Acid, 1.0N Solution
Attachments
Specifications
UPC:
724832080244
Maximum Age:
18+
Maximum Grade Level:
HS+
 No Choking Hazard
No Choking Hazard